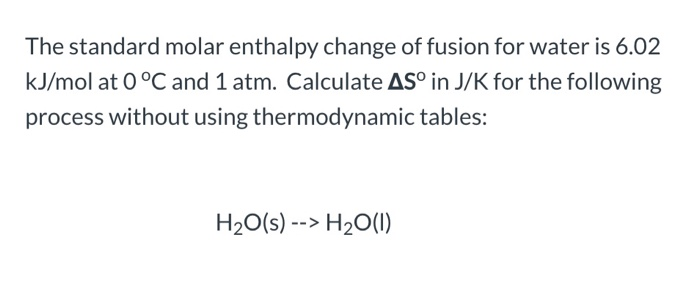
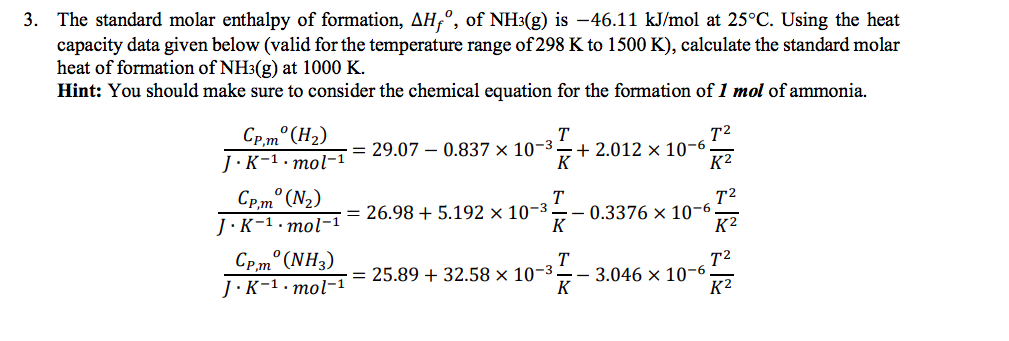
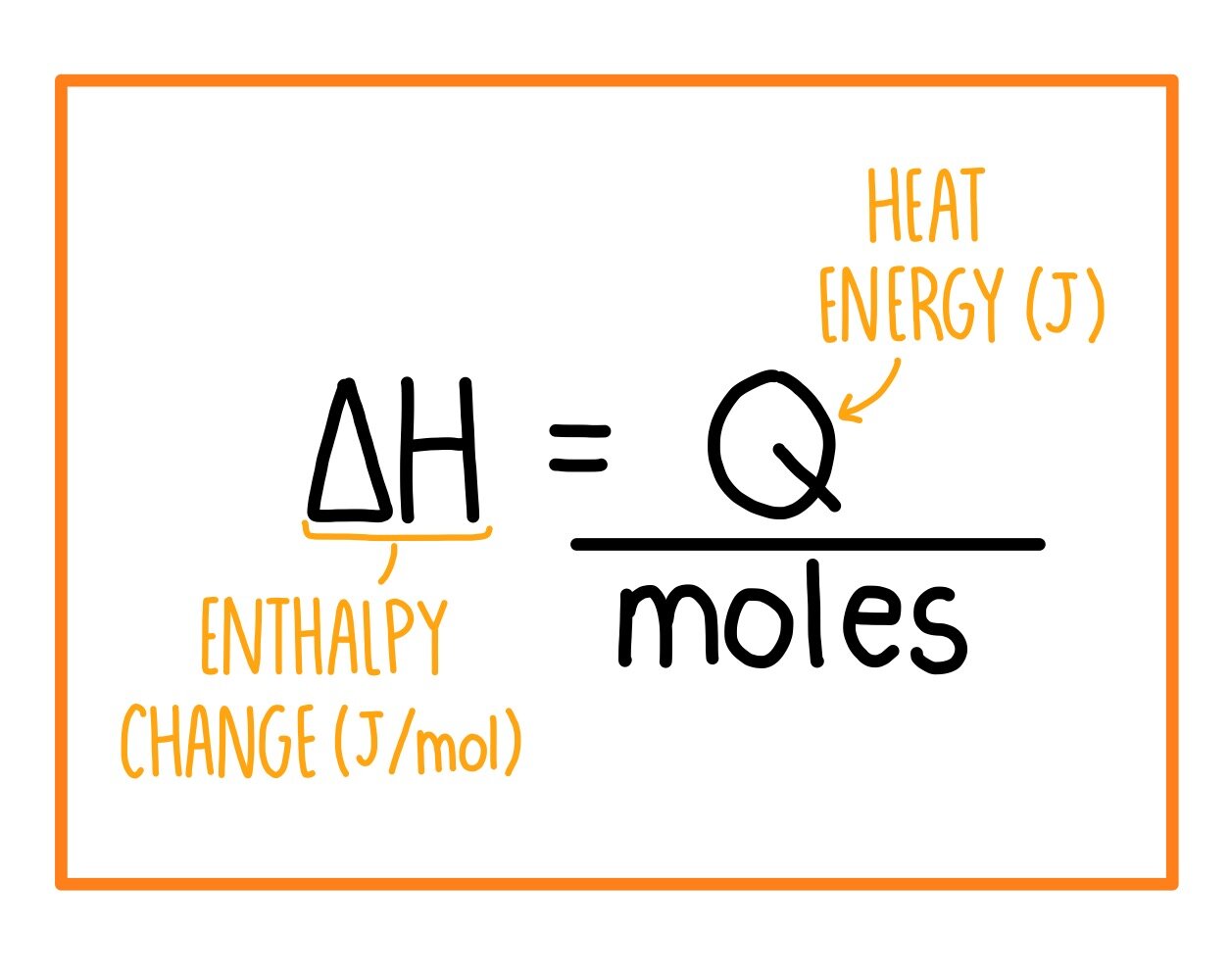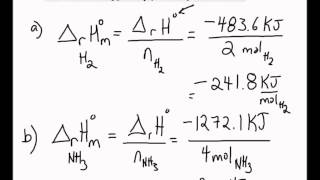Calculate the change in molar entropy and change in Gibbs' free energy when 1 mol of liquid butanol vaporizes at 25ºC to a gas that is at 0.001 atm? | Socratic
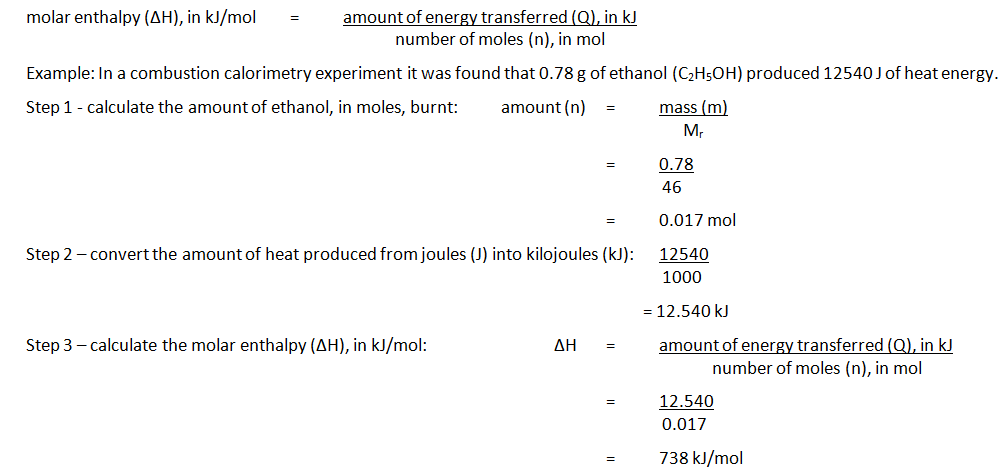
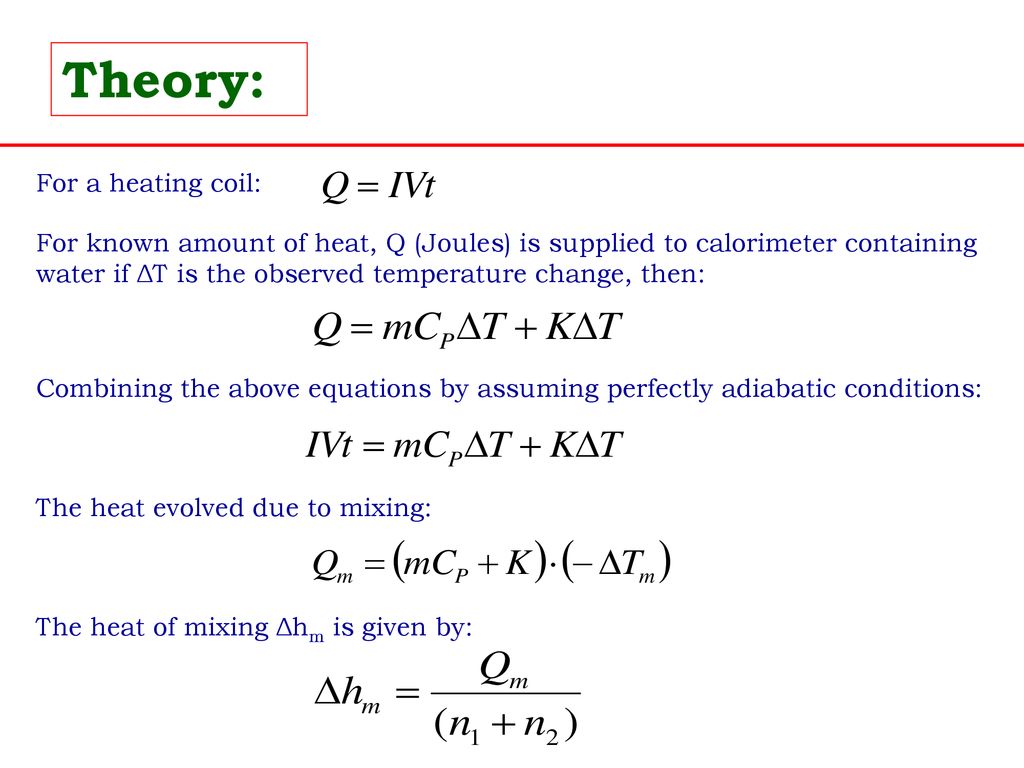
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q - TutorMyself Chemistry

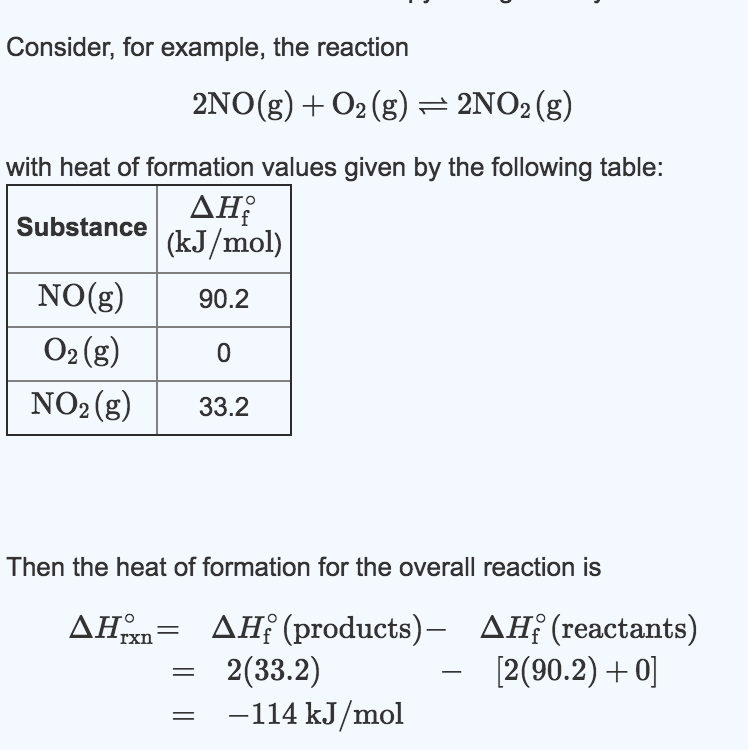
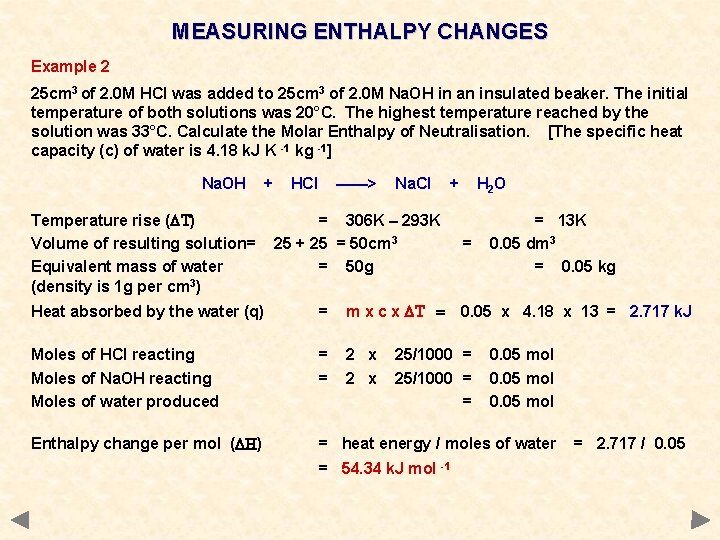
The molar enthalpy of neutralization was experimentary shown to be 51.5kJ per mole of 0.5M hydrochloric acid and 0.5M sodium hydroxide. If the volume of...

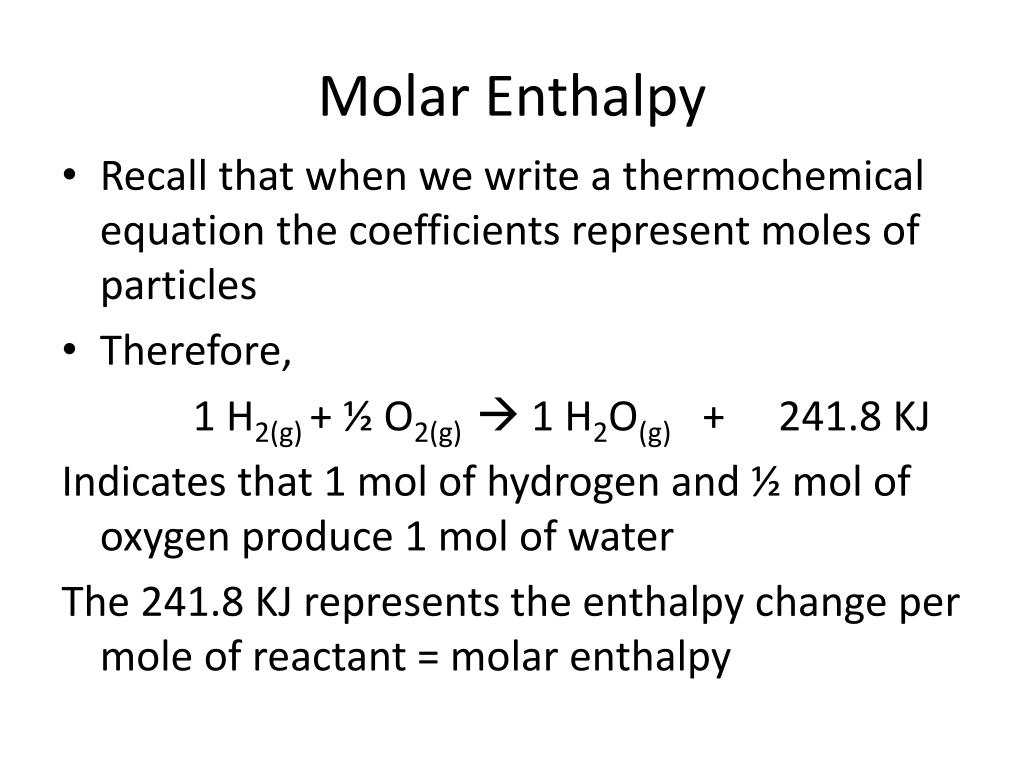
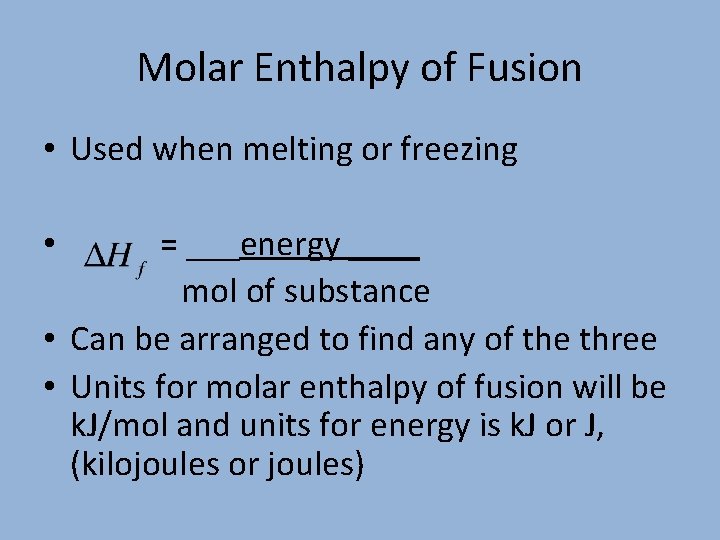
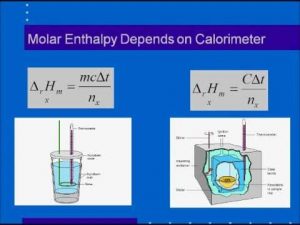
Molar Enthalpies. use proper scientific terminology to describe molar enthalpies calculate molar enthalpies Calculate molar enthalpies using the. - ppt download